Cosmetic ingredients
Safety & Efficacy: see our in vitro models
We combine cell culture, flow cytometry, and gene expression to prove the safety and effectiveness of your products.
All of our tests are available in the following in vitro models:
- Immortalized human keratinocytes (HaCaT)
- Normal Human Epidermal Keratinocytes (NHEK)
- Normal Human Dermal Fibroblasts (NHDF)
- Reconstructed Human Epidermis (RHE, RHE FT)
- Human Dermal papilla cells (HDPCs)
- Primary cell cultures (ocular tissue, adipose tissue, and umbilical cord tissue)
- Neoplasic cell lines (RAJI, breast and prostate cancer)
Safety assays
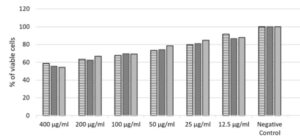
Acute toxicity tests evaluate the toxic potential of human cell culture. The method provides information on the hazardous properties and allows the substance to be classified for acute toxicity according to the Globally Harmonised System of classification and labeling of chemicals.
Techniques:
- Neutral red absorption
- Flow cytometry -apoptosis and late death
- MTT assay
- IC50 and LD50
Reference: OECD TG 129; OECD TG 420; OECD TG 423; OECD TG 425.
OECD 487
Genotoxicity tests are designed to detect compounds that induce genetic damage by different mechanisms.
- Fluorescence microscopy
- Flow cytometry
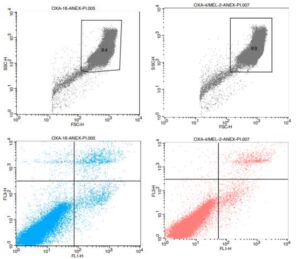
Evaluation of the potential of skin inflammation of the product. Skin inflammation safety testing and risk assessment for products and there ingredients are critical requirements before marketing.
Technique:
- Flow cytometry
- Gene expression (RT-qPCR)
Reference: Curr. Protoc. 2021 Mar;1(3):e72. DOI: 10.1002/cpz1.72.
OECD 439
Evaluation of the skin irritation potential in vitro after topical application of the investigational product in a three-dimensional model of RhE, composed of untransformed epidermal keratinocytes of human origin, being evaluated the cell viability by MTT.
OECD437
To evaluate irritation in rabbit corneal cell culture (SIRC) with two concentrations of the test substance (5% and 0.05%). After a brief exposure time a cell viability assay (MTT Test) is performed to measure the viability of the cells.
OECD442E
Skin sensitisation refers to an allergic response following skin contact with the tested chemical, as defined by the United Nations Globally Harmonized System of Classification and Labelling of Chemicals (UN GHS).
Technique: Flow Cytometry.
Identification of agents that cause structural chromosome aberrations in cultured mammalian somatic cells. Structural aberrations may be of two types: chromosome or chromatid.
Reference: OECD 473 and EC B.10 – “Chromosome aberration test in human cells”.
Evaluation of photo-cytotoxicity by the relative reduction in viability of cells exposed to the chemical in the presence versus absence of light.
Reference: OECD TG 432 – In Vitro Phototoxicity Test 3T3 NRU.
Microbiome evaluation test after application of a test cosmetic for 28 days, in three volunteers aged 18-45 years. The test formulation will be applied twice a day on the skin region (to be defined) of the volunteers. On day 0 and day 28, swab samples from the surface of the skin in the designated area will be collected. 16S rRNA sequencing analysis will identify the microbial diversity of the skin.
Reference: LEE, Hyo Jung; JEONG, Sang Eun; LEE, Soyoun; KIM, Sungwoo; HAN,Hyuntak; JEON,Che Ok. Effects of cosmetics on the skin microbiome of facial cheeks with different hydration levels. Microbiologyope
n, [S.L.], v. 7, n. 2, p. 00557-00561, 29 Nov. 2017.
Wiley.
Evaluation of developmental and reproductive toxicity. Provide information concerning the effects of prenatal exposure on the developing organism.
Reference: OECD 414 e EC B.31.
The test chemical is applied topically to a minimum of two three-dimensional Reconstructed human Cornea-like Epithelium (RhCE) tissue constructs and tissue viability is measured following exposure and a post-treatment incubation period. RhCE tissue viability is classically measured by enzymatic conversion of the vital dye MTT by the viable cells of the tissue into a blue MTT formazan salt that is quantitatively measured after extraction from tissues.
Test System: SkinEthicTM HCE / Human Corneal Epithelium.
Reference: OECD 492 – Reconstructed human Cornea-like Epithelium (RhCE) test method for identifying chemicals not requiring classification and labelling for eye irritation or serious eye damage.
Relate concentration or dose to the observed toxicity and to understand its mechanism of toxicity.
Reference: OECD 417 and EC B. 36.
OECD 471 – Bacterial Reverse Mutation Assay
Bacterial cells are exposed to test chemical in the presence and absence of metabolic activation, either by plate incorporation or by pre-incubation prior to plating out. Mutations are determined by scoring bacterial growth (revertant colonies) on selective agar plates lacking the essential amino acid.
Five different analysable concentrations of the test substance will be used.
The recommended combination of strains is:
- S. typhimurium TA1535;
- S. typhimurium TA1537;
- S. typhimurium TA98;
- S. typhimurium TA100;
- S. typhimurium TA102 or E. coli WP2 uvrA (pKM101)r.
Efficacy assays
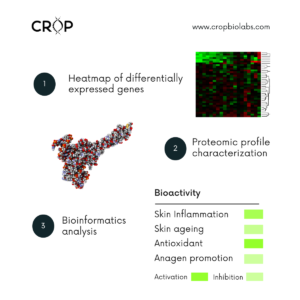
Bioactivity molecular screening can help you to know if your ingredient, extraction fraction, or compound can play more than one bioactive role in a cosmetic formulation. Through this test you can find new claims indicative in a fast a cost-effective way.
Technique: proteomic characterization, gene expression, and bioinformatics.
Exposure of test compound to fibroblast culture and quantification of AQP3, AQP9 and AQP10 gene expression (RT-qPCR).
Reference: Skin Res Technol 2017 Nov;23(4):486-490. doi: 10.1111/srt.12360.
Cells are exposed to the test product and the following extracellular matrix proteins are quantified by chemiluminescence metalloproteases MMP1, Elastin, and Collagen.
Technique:
- Phenotypic evaluation (collagen, elastin, HA, MMPs, versican);
- Oxidative damages (protein carbonylation, lipid peroxidation, protein glycation).
Reference: Toxicol Lett. 2016 Sep 30;259:60-68. doi: 10.1016/j.toxlet.2016.07.026.
The antioxidant potential (AOP) of the skin is known to be a decisive factor in skin aging. External aggressors such as stress or sun irradiation induce radicals and biochemical changes, thus affecting the AOP of cells and skin.
Technique: Gene expression (RT-qPCR): SOD1, CAT, GPx1, NRF2 genes.
Reference: Thermo Scientific Protocol: Antioxidant-Capacity-AN-VarioskanLUX-ORAC-E.
Exposure of Human Hair Papilla Capilar Cells (HDPCs) to the test products and measurement of angiogenesis growth factor (VEGF) in Flow Cytometry; and cell proliferation rate (MTT).
Technique:
- Angiogenesis in HHDPCs;
- B-catenin protein immunostaining, BrdU (bromodeoxyuridine) incorporation and proliferation (MTT).
Reference: J Dermatol Sci. 1994 Jul;7 Suppl:S55-72. doi: 10.1016/0923-1811(94)90036-1; BMC Dermatol. 2013 Oct 29;13:15. doi: 10.1186/1471-5945-13-15.

Kim D, Park S. Pharmacological therapeutics in androgenetic alopecia. Journal of the Korean Medical Association. 277-285, 2020.
Determination of antimicrobial efficacy for the strain associated with acnes. The active will be diluted in different concentrations in Müller–Hinton medium and the growth of the bacterial strain will be analyzed under aerobic or anaerobic conditions for P. acnes for 24-48 hours.
Technique:
- RT-qPCR for lipogenesis mRNA gene expression (ATGL, HSL, PRDM);
- 5-alpha Reductase (SRD5A1-3) activity and testosterone metabolism.
Reference: CLSI Standard M11, 9th ed ; Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobicall.
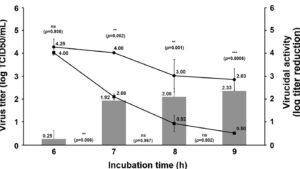
- EN14476:2019 – Quantitative suspension test for evaluation of virucidal activity in the medical area for hand sanitizers;
- EN 1276:2019 – Quantitative suspension test for the evaluation of bactericidal activity of chemical disinfectants and antiseptics.
Technique: Exposure test (TCID50 and CFU).
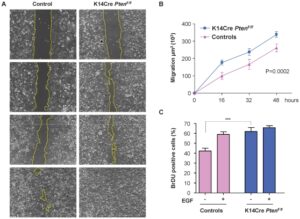
After growing fibroblasts in CytoSelect™ 24-Well Wound Healing Assay and exposure to the product, the wound area is quantified by imaging software for 15 days.
Technique:
- Cell cytotoxicity and proliferation (MTT, BrdU, NRU);
- Wound-healing (Scratch-assay).
Reference: NATURE PROTOCOLS | VOL.2 NO.2 | 2007
Human preadipocytes are incubated in an adipogenic differentiation medium along with the test product.
Reference: J Endocrinol 2008 Sep;198(3):459-69. doi:
10.1677/JOE-08-0264. Epub 2008 Jun25.24.
Assessment of effects on Lipogenesis through mRNA expression analysis of the ATGL, HSL, and PRDM genes by RT-qPCR.
Reference: Exp Clin Endocrinol Diabetes 2008 Apr;116(4):203- 10. doi:10.1055/s-2007 -993148. Epub 2007 Dec 10.
Melanocytes (B16) will be treated with three concentrations of non-toxic product test for a period of 48h. After that, they will be exposed to stress oxidative by UV or IL-1a. Afterward, it will activity quantification of tyrosinase and quantification of melanin.
Reference: Pereira AFC, Igarashi MH, Mercuri M, et al. Whitening effects of cosmetic formulation in the vascular component of skin pigmentation. J Cosmet Dermatol. 2019;00:1‐7.